Sulfate Water Structure My XXX Hot Girl
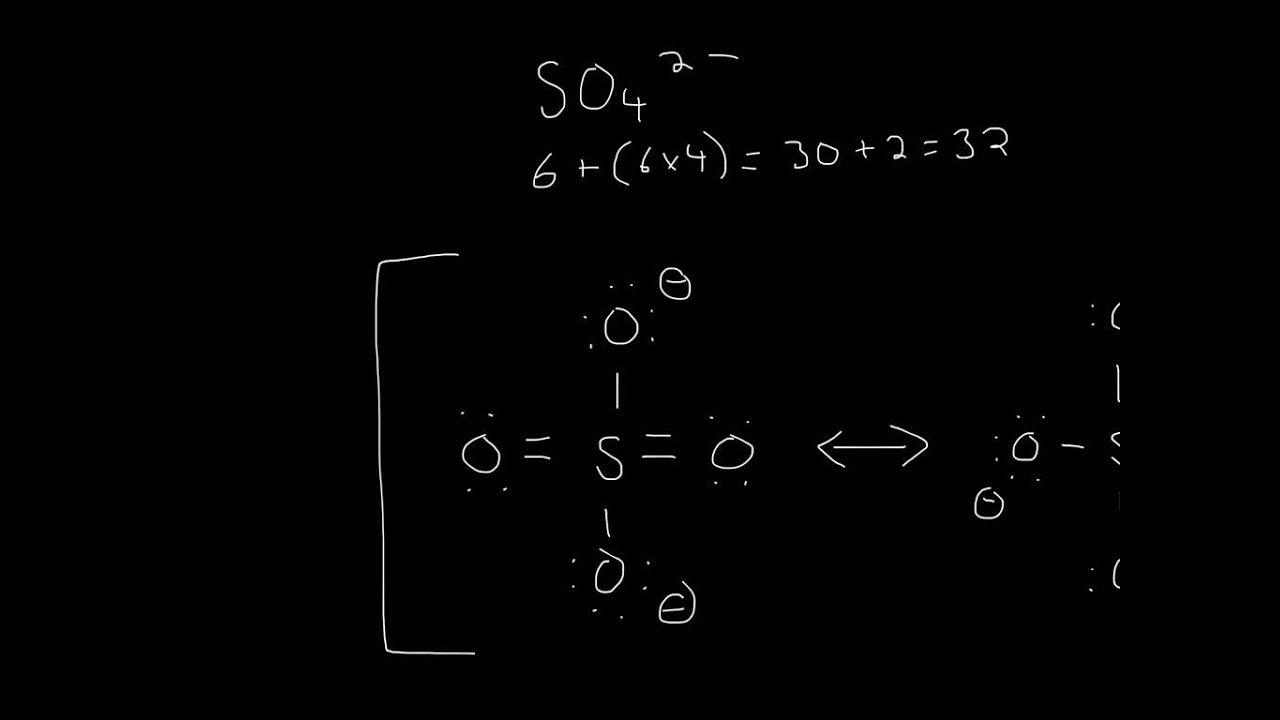
SO4 2 Resonance YouTube
Lewis Dot Structure of SO4 2- (Sulfate Ion) kentchemistry.com 25K subscribers Subscribe Subscribed 363 Share 196K views 12 years ago Every Video I quickly take you through how to draw the Lewis.

The shape of [Cu(NH3)4]SO4 is Chemistry Questions
1. The bromine atom has seven valence electrons, and each fluorine has seven valence electrons, so the Lewis electron structure is. Three fluorines are bonded to a central bromine. Each fluorine has three lone pairs, Bromine has two lone pairs. Once again, we have a compound that is an exception to the octet rule. 2.

Which of the following statements is true about [Cu(NH3)4] SO4
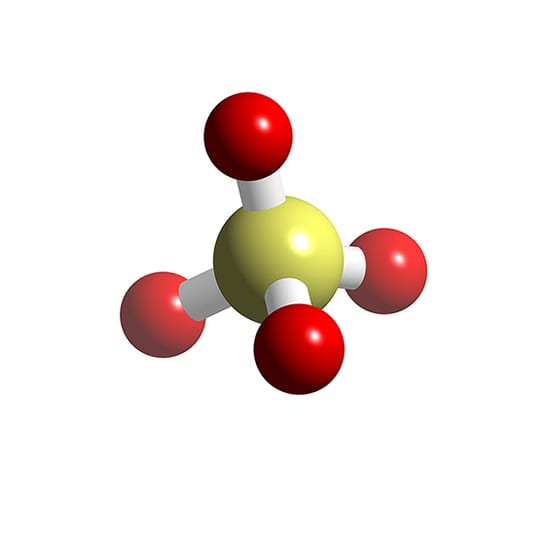
It comprises one Sulphur atom, four Oxygen atoms, and a charge of -2. It is a polyatomic anion and is used widely to synthesize other sulfates such as Zinc Sulfates, Magnesium sulfates, Iron sulfates, and much more. It is also a sulfate salt for sulphuric acids.
Sulfate Water Structure My XXX Hot Girl
We are now going to sketch the skeletal diagram of sulfate ion with the help of atomic symbols and dot line structures. Here, we have put the symbols of sulfur and oxygen as per notations and put the valence electrons as dots. Step 4: Check the Octet rule
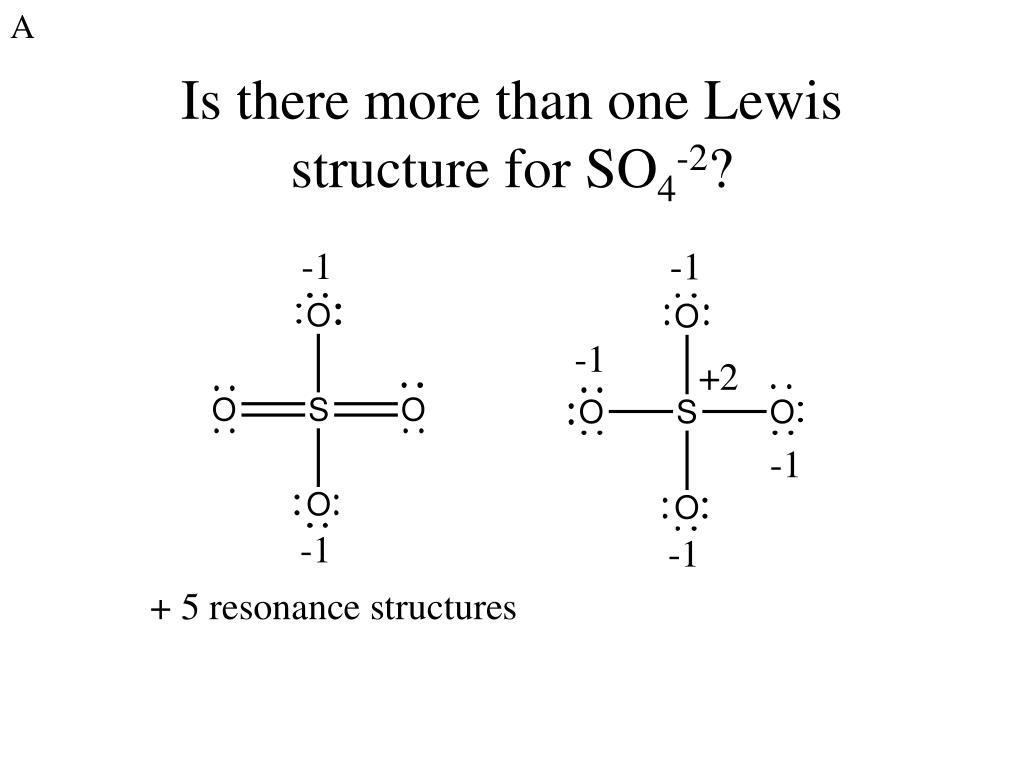
PPT Intersection 4 PowerPoint Presentation, free download ID6313576
This chemistry video explains how to draw the lewis structure of the sulfate ion SO4 2-.Chemistry - Basic Introduction: https://www.youtub.
Lewis结构,分子几何,杂交和极性技术科学家万博网页版 万博网页版,万博体育app手机版登录
Steps of drawing SO4 2- lewis structure Step 1: Find the total valence electrons in SO4 2- ion. In order to find the total valence electrons in SO4 2- ion, first of all you should know the valence electrons present in sulfur atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.). Here, I'll tell you how you can easily find the.

Lewis Dot Diagram For So4 2
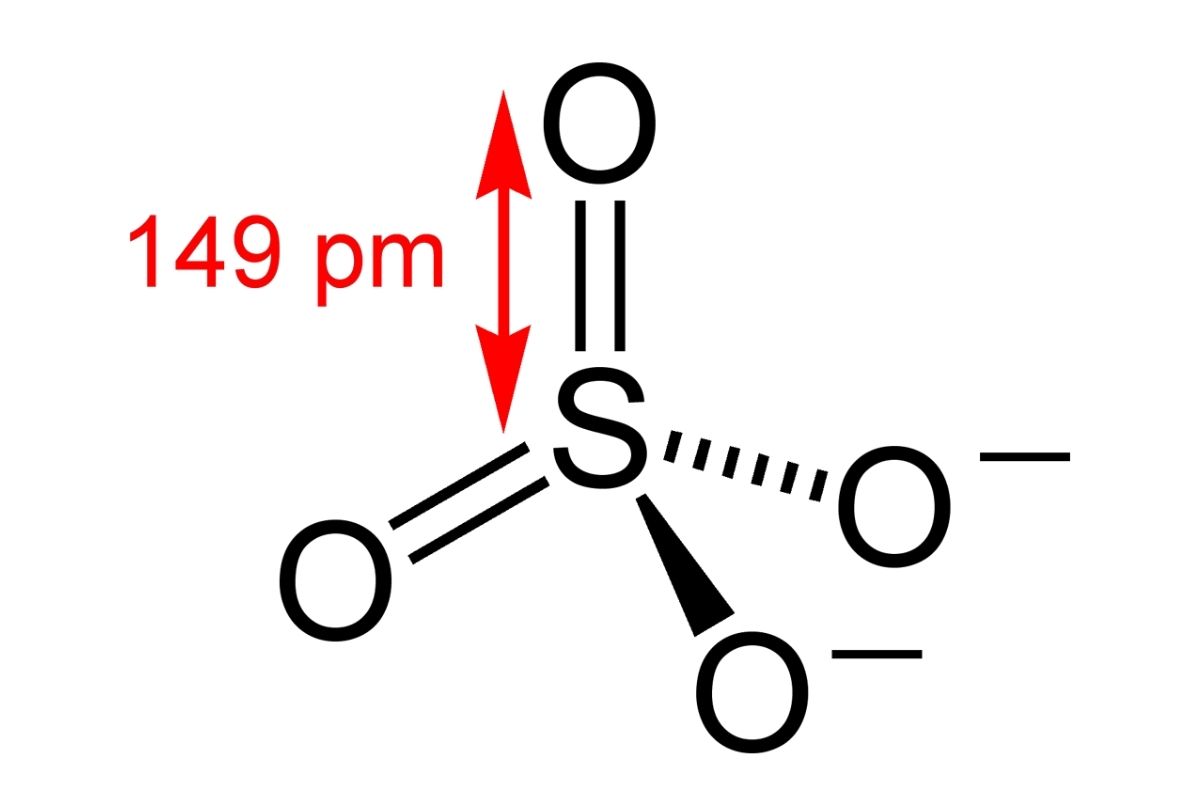
If we look at the structure or the shape of the molecule, it has a tetrahedral geometry which is further based on the VSEPR theory. In simple terms, sulphate ion has a star-shaped geometry. It is represented as follows: Structure of Sulphate The atoms are placed at a 109.5° angle.

Lewis Structure SO4 2 plus dipoles, shape, angles, resonance and formal charges YouTube
A step-by-step explanation of how to draw the Sulfate Ion Lewis Dot Structure (SO42- ). We'll also look at the molecular geometry, bond angles, electron geo.

draw the structure and predict the shape of SO4^2 Chemistry Chemical Bonding and Molecular
Let's do the SO4 2- Lewis structure, for the sulfate ion. On the periodic table: Sulfur, 6 valence electrons; Oxygen also has 6, we have 4 Oxygens, multiply by 4; and these 2 valence electrons up here, we need to add those, as well. That gives us a total of 32 valence electrons.

How To Draw The Lewis Structure of SO4 2 (Sulfate Ion) YouTube
Chemistry learning made easy.This tutorial will help you deal with the lewis structure and moleculargeometry of sulfate ion (SO4 2-)

H2SO4 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
A step-by-step explanation of how to draw the SO4 2- Lewis Dot Structure (Sulfate ion).For the SO4 2- structure use the periodic table to find the total numb.

How To Draw The Lewis Dot Structure For So4 2 (sulfate Ion) Youtube 1B5
Lewis Structure for SO. 4. 2-. (Sulfate Ion) We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and how it might interact with other molecules. -the physical properties of a molecule such as boiling point, surface tension, etc.
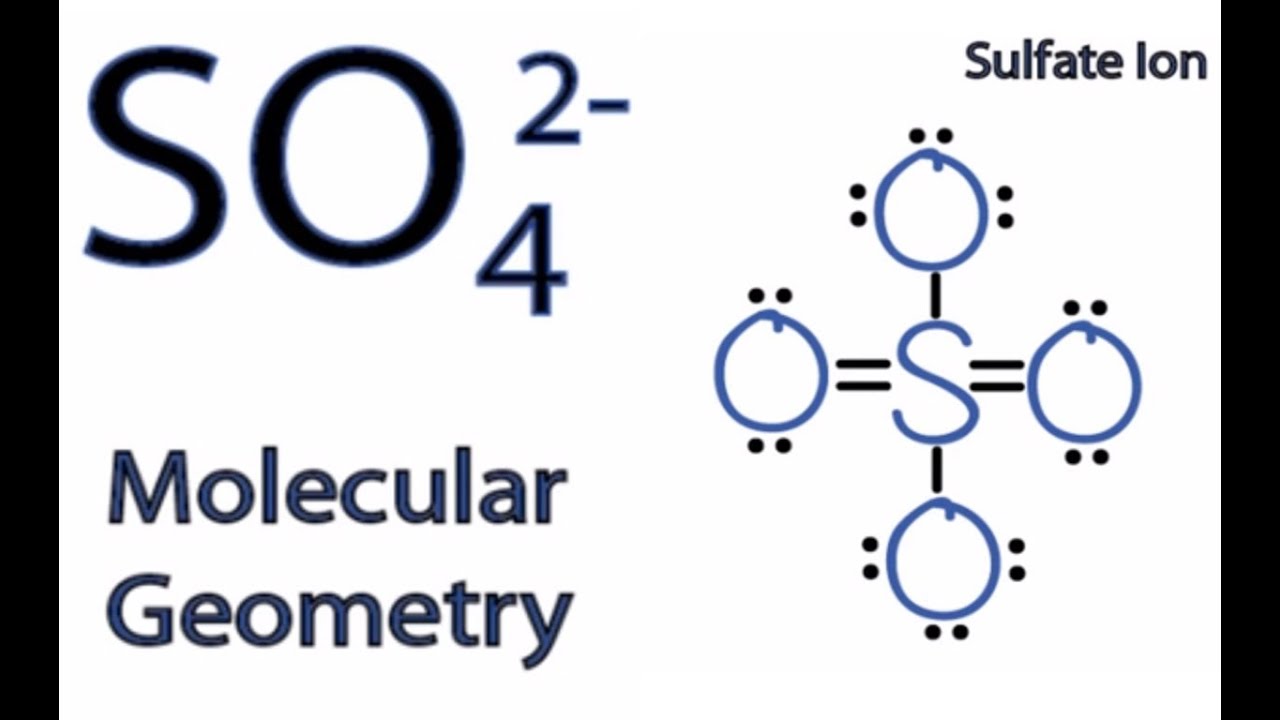
SO4 2 Molecular Geometry / Shape and Bond Angles YouTube
5 Steps to Draw the Lewis Structure of SO4 2- ion Step #1: Calculate the total number of valence electrons Here, the given ion is SO4 2-. In order to draw the lewis structure of SO4 2- ion, first of all you have to find the total number of valence electrons present in the SO4 2- ion.
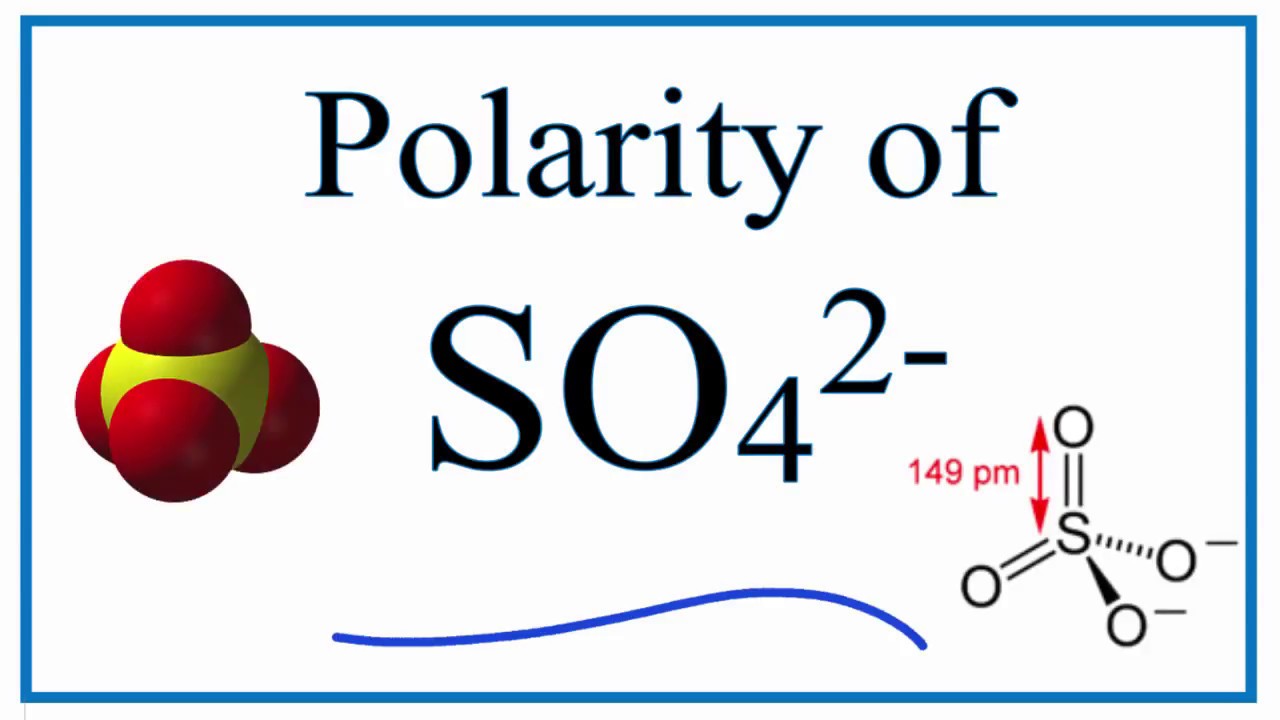
Is SO42 Polar or Nonpolar? (sulfate ion) YouTube
Ethyl Amine (D 2 O) Proton Exchange; Shapes of molecules VSEPR. H 2 O Water; NH 3 Ammonia; CH 4 Methane; SF 6 Sulfur Hexafluoride; SF 4 Sulfur Tetrafluoride; PF 5 Phosphorus Pentafluoride;. Home / Gallery / [SO4]2- - Sulfate [SO 4] 2-- Sulfate. CONTROLS () How useful was this page? Click on a star to rate it! Submit Rating . Average.

Chemistry Physical Chemistry stucture of SO4 askIITians
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, and.
Minimum bond angle is present in so3 so2 so4^2 so3^2
A quick explanation of the molecular geometry of SO4 2- including a description of the SO4 2- bond angles.Looking at the SO4 2- Lewis structure we can see th.